Our laboratory focuses on the application of biochemical methods in various scientific fields. We apply biochemical, molecular biological, transcriptomic, genomic, proteomic techniques for the study glycobiology of ticks and tick-borne pathogens, tick-host-pathogen interactions, nanostructured surface functionalization, and the study of fish development.
Research
Laboratory of Applied Biochemistry participated in the C4SYS research infrastructure.
C4SYS is a priority infrastructure project on the national roadmap, and currently builds on close collaboration between the Academy of Sciences (Institute of Nanobiology and Structural Biology, Nove Hrady, Institute of Microbiology, Prague, Global Change Research Center, Brno), the University of South Bohemia and Masaryk University, Brno.
Services offered by the laboratory in the frame of C4SYS can be found here.
Published papers
by the laboratory team members can be found here.
Research focus
A) Interaction of tick-borne encephalitis virus with tick and host on the molecular and cellular level.
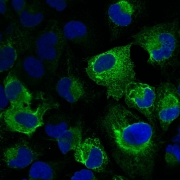
Tick-borne encephalitis virus is one of the most dangerous tick-transmitted pathogens in Europe and Asia. We aim to better characterize the replication of the tick-borne encephalitis virus, the interaction of viral proteins with host and tick molecules, the regulation of virus replication by the host anti-viral proteins on the molecular level, as well as the effects of viral replication on cellular mechanisms.
https://doi.org/10.1371/journal.pntd.0007745
https://doi.org/10.1099/jgv.0.000853
https://doi.org/10.1016/j.dib.2019.105029
https://doi:10.1016/j.ttbdis.2020.101420
https://doi.org/10.1016/j.csbj.2022.05.052
B) Tick research
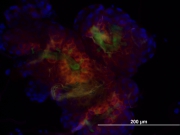
Fibrinogen-related proteins (FRePs) with lectin activity were studied in the Dermacentor and Rhipicephalus ticks. These proteins participate in the tick innate immune response. All of the proteins were found to be glycosylated and a cross-reaction of anti-FReP antibodies with the tick storage protein Hemelipoglycoprotein (HLGP) was discovered; however, the cross-reactivity is most probably dependent on the epitope similarity as sequence similarity was not found between fibrinogen and FRePs on one hand and HLGP on the other. The hemagglutination activity of Rhipicephalus ticks was inhibited by sialic acid and sialylated glycoproteins, GalNAc, and GlcNAc, suggesting similarity to another FReP from Ornithodoros moubata, the Dorin M protein. (Sterba et al., Parasite Vector 2011)
Mass spectrometric analysis of D. marginatus HLGP N-glycans in a collaborating Novotny laboratory (Indiana University, Bloomington, IN) showed the presence of high-mannose and complex type; paucimannosic type glycans were not observed. Furthermore, lectin-activity of HLGP was studied in collaboration with M. Wimmerova lab (Central European Institute of Technology, Masaryk University, Brno, Czech Republic). The highest binding activity was found for galactose. (Dupejova et al., Parasite Vector 2011).
Sialylated N-glycans were detected in Ixodes and Dermacentor ticks – both N-acetylneuraminic and N-glycosylneuraminic acid (NeuGc) were detected again in collaboration with Prof. Novotny's group in Bloomington, IN. Antibodies against NeuGc were utilized for tracking of sialylated glycans in tick tissues and thus we showed the route of sialylated host glycoproteins from the tick gut through the hemolymph to the salivary glands, where they are in part excreted back into the host. (Vancova et al., J. Insect Physiol. 2012).
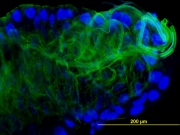
Next, we used a combination of sialic acid quantitation and detection of metabolically incorporated sialic acid (Click-iT chemistry) to determine expression of sialylated glycoproteins by the ticks themselves. Using this approach we showed, that majority of sialic acid present in fed female Ixodes ricinus ticks is coming from the host and not the tick itself. This can be one of the immune system evasion strategies employed by ticks (Sterba et al., Carbohydr. Res. 2014).
Recently, we work on the determination of the importance of glycan moieties (NeuAc and NeuGc) for the infection of tick and host cells by the Anaplasma marginale MSP1a in collaboration with Prof. de la Fuente's laboratory (IREC, Universidad de Castilla-La Mancha, Ciudad Real, Spain). While it was previously shown, that Anaplasma infection of tick cells is dependent on the presence of core-fucosylated N-glycans, here we are showing for the first time the importance of sialic acid for the infection of tick cells. The presence of host sialylated molecules in tick tissues and on the surface of tick cells could explain these findings.
Our current knowledge on the tick glycobiology was published recently. https://doi.org/10.1186/s13071-018-3062-7
https://doi.org/10.1186/s13071-019-3460-5.
https://doi.org/10.1038/s41598-020-70330-5
https://doi.org/10.1186/s13071-020-04173-4
Various aspects of gene expression regulation are studied in our laboratory, primarily changes in the gene expression in the various tick life-stages and methyltransferases responsible for DNA and RNA methylation.
https://doi.org/10.1016/j.ttbdis.2019.101348
C) Functionalized and nanostructured surfaces, biosensors
Antimicrobial effects on various modified and functionalized materials are studied in collaboration with our colleagues from the Laboratory of Applied Plasma Physics, Department of Physics. Furthermore, biocompatibility of the newly prepared surfaces for the growth of human cells is studied. It is important to mention, that we are entirely changing from the usually used cancer cell lines to primary human cells in this research field to better model the in vivo system.
http://dx.doi.org/10.1002/ppap.201900003
https://doi.org/10.1016/j.matlet.2018.07.082
https://doi.org/10.1016/j.apsusc.2019.07.135
https://doi.org/10.1007/s00216-019-02329-5
https://doi.org/10.1016/j.surfcoat.2020.125805
https://doi.org/10.1021/acsami.1c16930
https://doi.org/10.1021/acs.langmuir.1c01409
D) Fish gametes and fertilization
We participate in research oriented on various aspects of gametes development and preservation in collaboration with several laboratories from the Faculty of Fisheries and Water Protection.
https://doi.org/10.1007/s10695-018-0538-5
https://doi.org/10.1016/j.anireprosci.2018.03.025
https://doi.org/10.3390/ani9100753
https://doi.org/10.1111/raq.12355
https://doi.org/10.3390/ijms22115925
https://doi.org/10.3389/fmars.2021.736087
https://doi.org/10.1016/j.theriogenology.2019.02.029