Selfish Immune System

Department of Molecular Biology and Genetics
Selfish Immune System
Immune system is hierarchically above the rest of the organism in energy and nutrients allocation during immune challenge. Immune system produces signals that suppress activity of other tissues leaving thus energy to immune cells.
by Tomas Dolezal and Adam Bajgar - Department of Molecular Biology and Genetics
Immune response is energetically demanding process. Immune cells, upon activation, switch their metabolism to increased aerobic glycolysis to support rapid synthesis of macromolecules; this switch is associated with increased glucose consumption by immune cells. Increased aerobic glycolysis was originally described by Otto Warburg in cancer cells and it is now recognized as being common for proliferating cells (Vander Heiden 2009), for example during development (Tennessen 2011).
“Selfish immune system” is a theoretical concept recently articulated by Rainer Straub (Straub 2014) with inspiration of “selfish brain theory”. These concepts put brain and immune system hierarchically above the rest of the organism in allocating energy/nutrition. During the fight-or-flight response or trauma/infection, the organism vitally depends on either the central nervous system or the immune system and thus these organs are privileged in energy allocation.
According to Straub, insulin resistance, leading to lower consumption of glucose and hyperglycemia, is a physiological way for the brain or immune system to usurp energy/nutrition during acute stress from the rest of the organism because brain and immune cells themselves do not become insulin resistant. Chronic insulin resistance, caused by chronic inflammation or by chronic mental activation, then leads to various pathologies such as diabetes, obesity, metabolic syndrome or chronic inflammatory diseases.
Experimental evidence demonstrating the selfish behavior of immune system, especially during physiological reactions and not during disease state, is largely missing. We have recently found that immune cells release adenosine which causes a systemic metabolic switch leading to suppression of development. Results were published in PLoS Biology.
We use infection of fruit fly (Drosophila melanogaster) larvae by a parasitoid wasp Leptopilina boulardi (you can watch YouTube video with infection here). Wasp injects its egg into Drosophila larva and if larva does not destroy the egg, the emerged wasp larva consumes the host – the parasitoid can be seen as an alien in the insect world. However, the host larva can mount a robust immune response and destroy the egg before the alien emerges from the egg. The host must rapidly produce specialized immune cells called lamellocytes which will encapsulate the parasitoid egg and destroy it by melanization – a toxic reaction within the capsule.
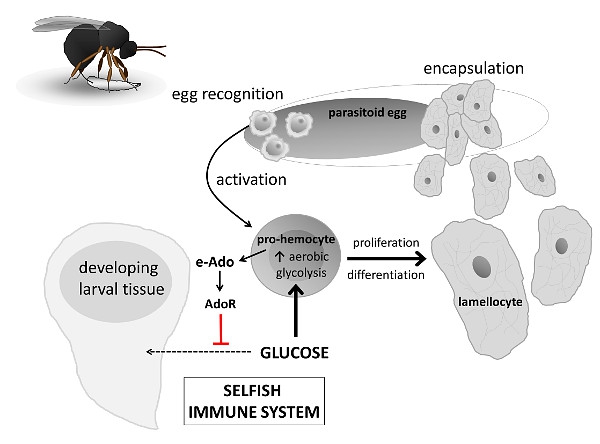
Fig 1. e-Ado-mediated systemic metabolic switch during parasitoid wasp infection of Drosophila larva.
Parasitoid egg is first recognized by circulating hemocytes which activate proliferation and differentiation of specialized immune cells, called lamellocytes, from pro-hemocytes. Lamellocytes eventually encapsulate and destroy the egg. Activated pro-hemocytes increase aerobic glycolysis, increasing thus significantly glucose consumption. They usurp the glucose from the rest of the organism by releasing adenosine. Extracellular adenosine (e-Ado) suppresses metabolism of other tissues by signaling via adenosine receptor (AdoR) and this slows down the larval development.
Such a robust immune response requires a significant amount of energy. Developing Drosophila larva obtains this energy by slowing down the development, leaving thus the energy to immune response. During the infection, activated immune system is allowed to behave selfishly. We have shown that activated immune cells, which must proliferate and differentiate into lamellocytes, produce adenosine that serves as a “selfish signal”. Extracellular adenosine (e-Ado) then suppresses the rest of the organism, leading to lower consumption of glucose by non-immune tissues and thus to a delay in development. This systemic metabolic switch is crucial for rapid production of lamellocytes and thus for effective immune response against the parasitoid egg. When we block adenosine transport from immune cells or when we block adenosine signaling, infected larva does not slow down the development, consumes the energy required by immune system and thus the resistance to parasitoid drastically drops. This represents a clear experimental evidence for a trade-off between development and immune response and a “selfish” behavior of immune system during stress. The selfishness of the immune system is crucial for effective immune response.
Bibliography:
- Bajgar A, Kucerova K, Jonatova L, Tomcala A, Schneedorferova I, et al. (2015) Extracellular Adenosine Mediates a Systemic Metabolic Switch during Immune Response. PLoS Biol 13(4): e1002135. doi:10.1371/journal.pbio.1002135
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324: 1029–1033.
- Straub RH. Insulin resistance, selfish brain, and selfish immune system: an evolutionarily positively selected program used in chronic inflammatory diseases. Arthritis Res Ther. 2014;16: S4.Tennessen JM, Baker KD, Lam G, Evans J, Thummel CS. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab. 2011;13: 139–148.
Read more …Selfish Immune System
- Hits: 556