Adam Bajgar group
- Hits: 4311
We combine cytogenetic and genomic approaches to study a role of genome organization and its changes in evolution.
RNDr. Martina Dalíková, Ph.D. (post-doctoral researcher)
Jana Štundlová. Ph.D. (post-doctoral researcher)
RNDr. Anna Voleníková (researcher)
M.Sc. Irena Provazníková (née Hladová; Ph.D. student)
M.Sc. Monika Kreklová (Ph.D.. student)
M.Sc. Jan Provazník (Ph.D. student)
B.Sc. Aneta Pilíková (M.Sc. student)
B.Sc. Monika Hrubá (M.Sc. student)
Kseniya Bobryshava (B.Sc. student)
Adriana Crhonková (B.Sc. student)
Karolína Petřvalská (B.Sc. student)
Pavel Hronek (B.Sc. student)
Tomáš Štrobl (B.Sc. student)
Read more …Laboratory of Chromosomics
In our newly established laboratory we focus not only on basic research but also on practical applications useful for everyday beekeeping. We investigate how bee nutrition and the way bees are managed by beekeepers influence the development of viral infections and other diseases. We consider thermoregulation as one of the key parameters that define the honey bees resistance to diseases.
Head of the laboratory: RNDr. Alena Krejčí, Ph.D.

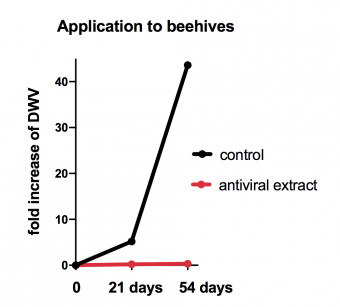
By testing a series of mushroom and herbal extracts we discovered substances that have a profound inhibitory effects on the loads of DWV virus in the honey bees. We tested these inhibitory properties in cage experiments in incubatotors but also in real beehives. Supplementation of these extracts to winter sugar feeding prevented increase in DWV loads in comparison to the control group. According to our preliminary results the natural extracts have no negative effects on the bee survival. The aim of our study is to use these extracts as a prevention of DWV spread in honey bees.

Pollen is critical for the development of honey bee brood and it is one of the key factors promoting the development of a healthy and numerous winter bee generation. However, the supply of pollen could often be limited in certain parts of the year. Using preferential tests we let the bees to decide the composition of a pollen subsitute that is rich in amino acids, vitamins and minerals, being similar to the natural pollen. An important part of the mixture is the Chlorella alage that resembles pollen by its composition and that the bees readily collect and consume. We showed that this nutritional supplement tripled the amount of brood in the beehives when offered to colonies in early spring in comparison to controls. We plan to enriche this nutritional supplement with one of our natural antiviral compounds to develop a balanced pollen substitute with antiviral properties.
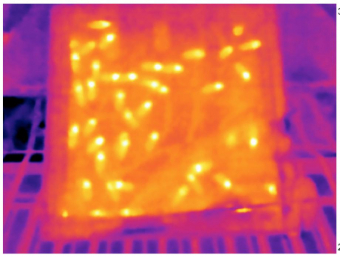
Honey bee brood develops optimally at temperature of 34-35°C, while the varroa mite prefers lower temperature of 32.6-33.5°C. Therefore the mites fertility is already compromised at the optimal bee brood temperature. Under longer exposures to heat shock temperatures above 42°C the mites die while the bees are minimally affected. In collaboration with the Department of embeded systems we developed sensitive thermosensors for monitoring temperature inside the brood cells. Our data indicate that the ability of bees to regulate brood nest temperature appears more flexible than anticipated.
Read more …Laboratory of Bee Biology
Research in the LEMDB is focussed on understanding the molecular mechanisms that underpin mammalian preimplantation embryo development; specifically the derivation of the first three cell lineages (trophectoderm, primitive endoderm & epiblast) that arise by the time of embryo uterine implantation. We are also investigating the fundamental processes that control the formation of viable mouse oocyte.
Head of the laboratory: prof. Alexander W. Bruce, Ph.D.
Read all about it!
Molecular mechanisms regulating preimplantation mouse embryo development and cell-fate
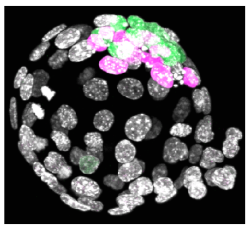
Following oocyte fertilization, the mammalian zygote undergoes a series of prolonged cell cycle divisions that results in the generation of the mature blastocyst consisting of three distinct cell types. The outside surface of the embryo comprises an epithelium of differentiated cells called the trophectoderm (TE – that subsequently develop to form the embryonic component of the placenta - see white nuclei opposite), whilst a second population of differentiated cells, the primitive endoderm (PE – that primarily give rise to cells of the yolksac - magenta stained nuclei), is found inside the embryo in contact with a fluid filled cavity and is distinct from other inner cells, the epiblast (EPI – that are the pluripotent progenitors cells of the foetus proper - green stained nuclei), that are completely buried inside the blastocyst structure.
The differentiated TE and PE cells eventually develop after implantation to support and direct development of the foetus that is itself derived from the EPI population of cells. However, the early mammalian embryo can exhibit extraordinary regulative behaviour and can successfully develop even when cells or added or removed. Despite this developmental flexibility, evidence is emerging that early inter-cell asymmetries are able to bias individual cell-fate in semi-predictable ways suggestive of at least some early patterning in the mammalian embryo.
Consequently, our primary research objectives are to uncover novel molecular mechanisms that impact upon successful blastocyst formation under both unperturbed and regulative conditions. Furthermore, to better understand how the blastocyst’s three constituent lineages are derived from a single totipotent zygote during the considerable spatio-temporal constraints of pre-implantation development and to probe the functional importance of very early inter-cell asymmetries.
We employ classical embryological and molecular biological techniques to probe the mechanisms of action of candidate cell fate influencing genes, identified in genomic scale expression screens of themouse embryo and mouse ES cells. Hence, we anticipate that our multi-disciplined approach, investigating the ‘in vivo’ balance between cell differentiation and retention of pluripotency in early mouse embryogenesis, will not only advance our fundamental knowledge of one of the most enigmatic periods of development but will also provide insight to clinical/ veterinary embryologists and those clinicians working in the field of regenerative stem cell-based medicine.
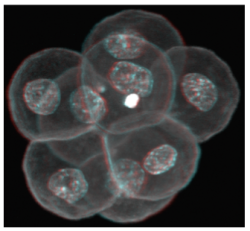
In female humans and mice the process of oocytogenesis is completed around the time of birth and the prevailing dogma dictates that no further primary oocytes are generated thereafter (in stark contrast to continuous spermatogenesis in males). The process of forming ootids (ootidogenesis) occurs during two rounds of meiosis and is initiated prenatally. However, ootidogenesis is stalled in the prophase stage of meiosis I so that by the time of birth the development of all the primary oocytes within the ovary is halted at this stage. Under the influence of the menstrual cycle hormones (follicle stimulating hormone and luteinizing hormone), meiosis I resumes in a sub-population of primary oocytes (and there is a time window in which sister chromosomes can recombine leading to genetic cross over). Ovulation then takes place and the haploid secondary oocyte plus the first polar body are generated, at which time meiosis II of ootidogenesis is initiated but is then arrested at the metaphase II stage. Upon sperm fertilisation, the secondary oocyte resumes meiosis II and extrudes the second polar body, thus forming the short-lived ootid, that then undergoes further maturation steps as a fertilised ovum, eventually forming a diploid zygote capable of further embryonic development.
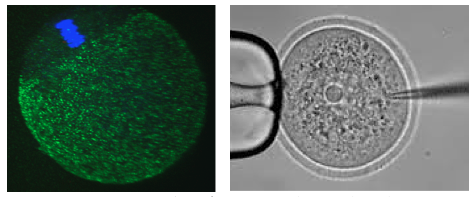
In our laboratory, we are interested in learning more about the molecular processes occurring when diploid primary oocytes are recruited into ootidogenesis and then mature into haploid secondary oocytes capable of being fertilised and supporting the preimplantation stages of embryonic development. We are particularly interested in the mechanisms that ensure the faithful segregation of chromosomes between the maturing oocyte and the extruded polar bodies (during meiosis I and II) and are functionally characterising a number of candidate target genes (using classical mouse genetics and RNAi/ mRNA microinjection and high resolution time-lapse microscopy techniques on oocytes maturing in culture).
Fluks M, Collier R, Walewska A, Bruce AW, Ajduk A. How great thou ART: Biomechanical properties of oocytes and embryos as indicators of quality in assisted reproductive technologies. Front. Cell Dev. Biol. in press (2024). IF2022 = 5.5.
Iyyappan R, Aleshkina D, Ming H, Dvoran M, Kakavand K, Jansova D, Del Llano E, Gahurova L, Bruce AW, Masek T, Pospisek M, Horvat F, Kubelka M, Jiang Z, Susor A. The translational oscillation in oocyte and early embryo development. Nucleic Acids Res. gkad996 (2023). IF2022 = 14.9
Gahurova L, Tomankova J, Cerna P, Bora P, Kubickova M, Virnicchi G , Kovacovicova K, Potesil D, Hruska P, Zdrahal Z, Anger M, Susor A and Bruce AW. Spatial positioning of preimplantation mouse embryo cells is regulated by mTORC1 and m7G-cap dependent translation at the 8- to 16-cell transition. Open Biology 8:230081 (2023). IF2022 = 5.800
Bora P , Gahurova L, , Hauserova A, Stiborova M, Collier R, Potěšil D, Zdráhal Z and Bruce AW. DDX21 is a p38-MAPK sensitive nucleolar protein necessary for mouse preimplantation embryo development and cell-fate specification. Open Biology 7:210092 (2021). IF2021 = 6.411 .
Bora P , Gahurova L, Mašek T, Hauserova A, Potěšil D, Jansova D, Susor A, Zdráhal Z, Ajduk A, Pospíšek M and Bruce AW. p38-MAPK-mediated translation regulation during early blastocyst development is required for primitive endoderm differentiation in mice. Commun. Biol. 4:788 (2021). IF2021 = 6.268
Virnicchi G, Bora P, Gahurova L, Šušor A and Bruce AW. Wwc2 is a novel cell division regulator during preimplantation mouse embryo lineage formation and oogenesis. Front. Cell. Dev. Biol. 8:857. (2020). IF2020 = 6.684
Del Llano E, Masek T, Gahurova L, Pospisek M, Koncicka M, Jindrova A, Jansova D, Iappan R, Roucova K, Bruce AW, Kubelka M and Susor A. Age-related differences in the translational landscape of mammalian oocytes. Aging Cell e13231 (2020). IF2020 = 9.304
Masek T, Del Llano E, Gahurova L, Kubelka M, Susor A, Roucova K, Lin C-J, Bruce AW and Pospisek M. Identifying the translatome of mouse NEBD-stage oocytes via SSP-profiling; a novel polysome fractionation method. Int. J. Mol. Sci. 21(4) 1254 (2020). IF2020 = 5.923
Bora P, Thamodaran V, Šušor A and Bruce AW. p38-mitogen activated kinases mediate a developmental regulatory response to amino acid depletion and associated oxidative stress in mouse blastocyst embryos. Front. Cell. Dev. Biol. 7:276 (2019). IF2019 = 5.186
Koncicka M, Tetkova A, Jansova D, Del Llano E, Gahurova L, Kracmarova J, Prokesova S, Masek T, Pospisek M, Bruce AW, Kubelka M and Susor A. Increased expression of maturation Promoting Factor components speeds up meiosis in oocytes from aged females. Int. J. Mol. Sci. 19(9), 2841 (2018). IF2018 = 4.183
Mihajlović AI and Bruce AW. The first cell-fate decision of mouse preimplantation embryo development: integrating cell position and polarity. Open Biology. 7(11) /rsob.170210 (2017). IF2017 = 3.286
Koštál V, Štětina T, Poupardin R, Korbelová J, Bruce AW. Conceptual framework of the eco-physiological phases of insect diapause development justified by transcriptomics profiling. Proc. Natl. Acad. Sci. USA. 114(32), 8532-8537 (2017). IF2017 = 9.504
Thamodaran V and Bruce AW. p38 (Mapk14/11) occupies a regulatory node governing entry into primitive endoderm differentiation during preimplantation mouse embryo development. Open Biology. 6(9) /rsob.160190 (2016). IF2016 = 3.481
Mihajlović AI and Bruce AW. Rho-associated protein kinase regulates subcellular localisation of Angiomotin and Hippo-signalling during preimplantation mouse embryo development. Reprod. Biomed. Online. 33(3), 381-390 (2016). IF2016 = 3.249
Mihajlovic AI, Thamodaran V and Bruce AW. The first two cell-fate decisions of preimplantation mouse embryo development are not functionally independent. Scientific Reports 13(5): 15034 (2015). IF2015 = 5.228
2020-2022: Czech Science Foundation (CSF): 21-03305S: ‘Characterisation of Hippo-signalling related genes during mouse oocyte maturation, acentrosomal cell division & blastocyst cell lineage allocation.’ Duration: 3 years. Finance: 8,672,000 CZK.
2018-2020: Czech Science Foundation (CSF): 18-02891S: ‘Regulating the balance between differentiation and pluripotency; molecular characterisation of p38-MAPK function in mouse blastocyst ICM maturation.’ Duration: 3 years. Finance: 6,247,000 CZK.
2017-2021: Marie Curie Career Individual Fellowship Grant (awarded jointly with a post-doctoral researcher, Lenka Gahurová Ph.D., who is hosted in my group; H2020): OOCSOCS: ‘Socs3 gene in oocyte maturation and fertilsation – a novel link between inflammation and infertility’. Duration: 3 years. Finance: 142, 720 EUR - reinstated after maternity leave (LG).
2013-2016: Czech Science Foundation (CSF): 13-03295S: ‘Functional characterisation of identified novel candidate cell-fate influencing genes during pre-implantation mouse development’. Duration: 4 years. Finance: 6,889,000 CZK.
2011-2015: Marie Curie Career Integration Grant (within the 7th European Community Framework Programme): IDNOVCELFAT2011: ‘Identification and characterization of novel cell-fate influencing genes in pre-implantation mouse development’. Duration: 4 years. Finance: 100,000 EUR.
The Visegrád Group Society for Developmental Biology (V4SDB) 
The LEMDB is an active supporter of the Visegád Group Society for Developmental Biology (V4SDB) with Alexander W. Bruce serving as a founder member of organising committee. The V4SDB is committed to supporting excellence in the field of developmental biology research throughout the Czech Republic, Poland, Hungary and Slovakia and is actively engaged in outreach and other collaborative efforts. See the V4SDB homepage http://v4sdb.org/index
The Inaugural Meeting of the V4SDB was held in the Czech city of Brno from 7-9th September 2018. You can find more details about the society and the Brno meeting on conference webpage (https://webcentrum.muni.cz/visegrad2018) or the V4SDB Facebook page: www.facebook.com/V4SDB
The Second V4SDB Meeting (COVID-19 delayed) took place in Szeged (Hungary) from 2nd-5th September 2021 -You can find more details about the Szeged meeting on the conference webpage (https://www.v4sdbszeged.com/) or the V4SDB Facebook page: www.facebook.com/V4SDB
The Third V4SDB Meeting was held in Warsaw (Poland) from 8th-10th September 2023 - You can find more details about the Szeged meeting on the conference webpage (https://v4sdb2023.pl/welcome_message) or the V4SDB Facebook page: www.facebook.com/V4SDB
The Fourth V4SDB Meeting is due to be held in Warsaw (Poland) in September 2025 - more details to follow: https://www.v4sdb.org/2023-v4sdb-meeting-warsaw-1-1
Laboratory alumni (Where are they now?)
Former post-doctoral researchers
Lenka Gahurová (2023 – former Marie Curie Individual Grant Fellowship holder): Faculty member and Assistant Professor at the University of South Bohemia in České Budějovice (Department of Molecular Biology & Genetics – Laboratory of Mammalian Epigenetics and Bioinformatics), Czech Republic.
Ender Yalcinkaya (2013): Eurofertil In Vitro Fertilization Center, Kocaeli University IVF unit, Embryology Laboratory, Istanbul, Turkey.
Former Ph.D. students:
Pablo Bora (2021): University of Massachusetts Medical School - laboratory of prof. Oliver J. Rando - https://www.umassmed.edu/randolab/
Giorgio Virnicchi (2021): PLAISANT, Rome, Italy - Transgenic Mouse Generation outsourcing company - http://www.plaisant.eu/
Aleksandar Mihajlović (2017): Centre de Recherche du Centre Hospitalier de l’Université de Montréal, Montréal, Canada (recipient of FRQS post-doctoral fellowship - 2019) - https://www.fitzharrislab.com/
Vasanth Thamodaran (2016): Tata Institute for Genetics and Society - TIGS, Bengaluru, India (recipient of INSPIRE Faculty Fellowship/ Early Career - Indian National Academy of Sciences - 2020) - https://tigs.res.in/people/scientists/vasanth-thamodaran/
Former Master's students:
Valeriy Kutsnya (2023): Ph.D. student: Research Centre at the University of Montreal Hospital CRCHUM (Canada; Prof. Greg Fitzharris, Ph.D.).
Pavlína Černá (2021): Pronatal Repro Human IVF clinic in České Budějovice (Budweis), Czech Republic.
Miriama Pekl’anská (2021): Ph.D. student: Institute of Parasitology, (AV ČR - Budweis); Laboratory of Molecular Biology of Ticks (RNDr. Radek Šíma, Ph.D.)
Michaela Kubíčková (2018): Czech TEAM Olympic Beach Volleyball Athlete.
Read more …Laboratory of Early Mammalian Developmental Biology (LEMDB)
Regulation of energy distribution into different organs is crucial for the organism. Immune response requires increased amount of energy/nutrition and overall metabolism adjustment is thus necessary. We are interested in an inter-organ communication which ensures proper energy re-distribution during immune response in Drosophila melanogaster model.
Head of the laboratory: doc. Mgr. Tomáš Doležal, Ph.D.
Labmanager: Lucie Hrádková
Research assistant: Mgr. Lenka Chodáková, Ph.D.
Postdocs: Dr. Ellen McMullen, rer. nat.
Students: Mgr. Pavla Nedbalová
Mgr. Tereza Dolejšková
Vojtěch Černý
Jakub Sysel
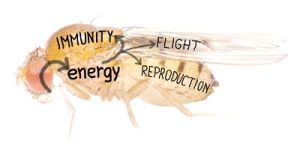

We employ three different types of infection in our experimental models – ① parasitoid wasp Leptopilina boulardi infection of Drosophila larvae, ② extracellular bacteria Streptococcus pneumonia infection of Drosophila adult fly causing an acute infection and ③ intracellular Listeria monocytogenes infection of adult fly causing a chronic infection. We analyze tissue-specific gene expressions and signaling, metabolites, effects on development and behavior throughout the infection and test impacts of various genetic manipulations on systemic physiology.
We have recently found that a systemic metabolic switch, which changes flow of energy from development towards immunity during immune response, is mediated by extracellular adenosine (e-Ado):
e-Ado is released from immune cells, which are activated to proliferate and differentiate into specialized immune cells - lamellocytes - upon the parasitoid wasp attack. Lamellocytes production requires energy, which is obtained by slowing down the host development. The switch from development to immune response requires e-Ado as a mediator. By releasing e-Ado, immune cells are able to usurp energy from the rest of the organism. This experimentally demonstrates a selfish (or privileged) behavior of immune system, which is hierarchically above all the other organs in the organism during immune challenge; theoretical concept of selfish immune system in humans was recently proposed by Dr. Reiner Straub.
Extracellular adenosine (e-Ado) is an important regulatory molecule with a low physiological concentration that can rapidly increase during tissue damage, inflammation, ischemia or hypoxia. During hypoxia or cellular metabolic stress, adenosine can be transported by nucleoside transporters to extracellular space from cells where its concentration raises up due to excessive breakdown of ATP. e-Ado then informs the surrounding tissues about the metabolic state of the cells/tissues via adenosine receptors. This can lead to various responses as vasodilation, increase of blood flow and therefore substrate delivery or it can stimulate glycogenolysis, providing energy substrates from stores to overcome stress. Increased e-Ado may also lead to a suppression of metabolic processes in certain tissues to conserve overall energy.
Additional information about e-Ado and details of our previous studies of e-Ado role in flies can be found in Tomas Dolezal's habilitation thesis.
Is it possible that adenosine plays similar role during immune response in humans? You can read editorial Adenosine: a selfish-immunity signal? on this topic.
Immune cells can suppress their own privilege behavior
The privilege of the immune system is vital in acute threats to the organism, but sooner or later it is necessary to dampen this behavior again so that privilege does not become selfish (as is the case with immunity that has been activated for too long) and the organism is not exhausted. How this is ensured showed our next model of activation of the immune response, namely bacterial infection of adult flies. We inject a precisely defined amount of bacteria (e.g. streptococcus or listeria) into the fly body. In studying these reactions, we found that the immune cells themselves again produce an enzyme at a later stage of the immune reaction, which reduces the amount of adenosine and thus suppresses its effects on the energy metabolism of the fly. When we genetically suppressed the functioning of this enzyme, it might help the fruit fly to fight streptococcus in the short term, but at the expense of greater depletion of energy reserves. In the longer term, however, it was rather harmful to flies, and in chronic listeria infection, it led to a shorter life and, on the contrary, benefited bacteria that probably got more nutrients at the expense of the host.
The results of this work were published in PLoS Pathogens:
Read more …Laboratory of Molecular Integrative Physiology in Drosophila