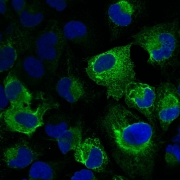
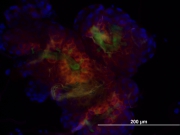
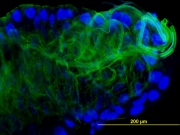
Macromolecular X-ray Crystallography is a technique used to study biological molecules such as proteins, viruses and nucleic acids (RNA and DNA) to an atomic resolution. This high resolution helps elucidate the detailed structure-based mechanism by which these macromolecules carry out their functions in living cells and organisms.

Laboratory description:
The Laboratory of Structural Chemistry consists of three sub laboratories and one training part:
MolBiol - is the laboratory of molecular biology designed for isolation and purification of proteins,
XtallExp – is the laboratory for crystallization experiments aimed at production of diffractable crystals. Obtained crystals are measured at the synchrotron radiation sources and diffraction data are used for solving of protein structure,
MolStruct – is the lab for molecular modeling and structural studies following by molecular dynamics studies,
XtallTraining – offers other colleagues with the interest of crystallization of proteins possibility to crystallize own proteins by themselves or with the help of our specialists
Key activities:
- basic research in the field of isolation and purification of proteins and their crystallization
- solving of protein structures from diffraction data and structural studies of studied proteins
- development and testing of alternative and advanced crystallization techniques
- close cooperation with affiliated laboratories in the Czech Republic and abroad
- training of others about crystallization of own proteins
- teaching of Inorganic chemistry 1 (UCH/100), Inorganic chemistry 2 (UCH/101), Organic chemistry for biology (UCH/032) a Biocrystallization methods (UCH/253) in czech
- organization of international courses under auspices of FEBS named FEBS-INSTRUCT practical crystallization course „Advanced methods in macromolecular crystallization“
International cooperation:
Prof. Joseph Ng team University of Alabama in Huntsville, USA in the Department of Biological Science.
Dr. math. et dis. nat. Jeroen R. Mesters – a senior Researcher and Lecturer, Deputy of Universität zu Lübeck, Institut für Biochemie at Lübeck, Germany.
Dr. Monika Budayova-Spano – Assistant Professor – HDR, University Joseph Fourier Grenoble, Institut De Biologie Structurale, Umr5075 CEA-CNRS-UJF, Grenoble, France
Dr. José A. Gavira-Gallardo – Research Scientist of Laboratorio de Estudios Cristalográficos
Instituto Andaluz de Ciencias de la Tierra, (CSIC-UGR) Armilla, Granada, Spain
Dr. Pavlina Rezacova a head of the Senior Research Group of Structural Biology at Institute of Organic Chemistry and Biochemistry AS CR
Doc. Radka Chaloupková, Ph.D. - Research Scientist at Loschmidt Laboratories, Department of Experimental Biology and Research Centre for Toxic Compounds in the Environment RECETOX, Masaryk University, Brno, Czech Republic
Infrastructure:



Fully-equipped biochemical laboratories and Laboratory of Crystallogenesis and Macromolecular Crystallography (Assoc. Prof. Ivana Kuta-Smatanova, PhD) at University of South Bohemia (Faculty of Sciences, Ceske Budejovice), well-appointed with crystallization robotic system Oryx 3, Dynamic Light scattering instrument, analytical balances, IWA distiller for redistilled water, deep-freeze, iceboxes, cold-room, incubators, ice generator, pH-meters, SDS-PAGE and horizontal electrophoresis, Western blot system, PCR thermocycler, ultracentrifuge Beckman, low speed cooled centrifuges, table centrifuges, spectrophotometer, cells disintegrator, high-magnificence stereomicroscopes, photocameras, Eppendorf pipette sets, commercial crystallization and molecular kits, chemicals, crystallization plates and all necessary plastic and glass for the cultivation, biochemical and crystallization experiments.
Computational laboratory is well equipped by international standards for computational chemistry (Cluster of the Beowulf type with nodes connected by Myrinet, Graphics work stations with 3D, 2 computer labs, licenses for Gaussian09, Amber10, Gromacs, modeler, Yasara, autodock etc.) as well fully equipped lab to perform all tasks of structural analysis of biological macromolecules by X-ray crystallography with computational hardware and software necessary for the diffraction data processing, structure determination, macromolecular structure refinement, validation and analysis (XDS, XDSapp, CCP4 program package, Coot, PyMol, IMosflm and others).
For the diffraction quality crystals could be tested at home X-ray diffractometer D8 Venture source from Bruker at Institute of Institute of Nanobiology and Structural Biology, Institute of Microbiology, Academy of Sciences of the Czech Republic and later on will be prepared for the data collection experiments on the synchrotron. Diffraction measurements could be performed on synchrotron macromolecular beamlines BESSY (Berlin, Germany), DEZY (Hamburg, Germany) or ESRF (Grenoble, France) following standard application procedures.
Publications
2020
Brodsky K; Kutý M; Pelantová H; Cvačka J; Rebroš M; Kotik M; Kutá Smatanová I; Křen V; Bojarová P: Dual Substrate Specificity of the Rutinosidase from Aspergillus niger and the Role of Its Substrate Tunnel. Int. J. Mol. Sci. Volume 21, Issue 16, 5671 (2020). https://doi.org/10.3390/ijms21165671
Marek M., Chaloupkova, R., Prudnikova, T., Sato, Y., Rezacova, P., Nagata, Y., Kuta Smatanova, I., Damborsky, J.: Structural and catalytic effects of surface loop-helix transplantation within haloalkane dehalogenase family. Computational and Structural Biotechnology Journal 18, 1352-1362 (2020). https://doi.org/10.1016/j.csbj.2020.05.019
Kuta Smatanova I.: Data analytics for protein crystallization, Crystallography Reviews 26, 1, 58-61 (2020). DOI: 10.1080/0889311X.2019.1691174
2019
Aryafard M, Jahanshahi M, Harifi-Mood AR, Minofar B and Kuta Smatanova I.: J. Chem. Eng. Data 64 (12), 5755–5764 (2019). https://doi.org/10.1021/acs.jced.9b00719
Chrast, L., Tratsiak, K., Planas-Iglesias, J., Daniel, L., Prudnikova, T., Brezovsky, J., Bednar, D., Kuta Smatanova, I., Chaloupkova, R. and Damborsky, J.: Deciphering the Structural Basis of High Thermostability of Dehalogenase from Psychrophilic Bacterium Marinobacter sp. ELB17. Microorganisms 7, 498 (2019). doi:10.3390/microorganisms7110498 www.mdpi.com/journal/microorganisms
Havlickova, P., Brinsa, V., Brynda, J., Pachl, P., Prudnikova, T., Mesters, J. R., Kascakova B., Kuty, M., Pusey, M.L., Ng, J.D., Rezacova, P. and Kuta Smatanova, I.: A novel structurally characterized haloacid dehalogenase superfamily phosphatase from Thermococcus thioreducens with diverse substrate specificity. Acta Crystallographica Section D: Structural Biology, D75(8), 743-752 (2019). https://doi.org/10.1107/S2059798319009586
Prudnikova, T., Kascakova, B., Mesters, J. R., Grinkevich, P., Havlickova, P., Mazur, A., Shaposhnikova A., Chaloupkova, R., Damborsky, J., Kuty, M. and Kuta Smatanova, I.: Crystallization and crystallographic analysis of a Bradyrhizobium elkanii USDA94 haloalkane dehalogenase variant with an eliminated halide-binding site. Crystals 9(7), 375 (2019). https://doi.org/10.3390/cryst9070375, https://www.mdpi.com/2073-4352/9/7/375
Tratsiak, K., Prudnikova, T., Drienovska, I., Damborsky, J., Brynda, J., Pachl, P., Kuty, M., Chaloupkova, R., Rezacova P., and Kuta Smatanova I.: Crystal structure of cold-adapted haloalkane dehalogenase DpcA from Psychrobacter cryohalolentis K5. Acta Crystallographica Section F-Structural Biology Communications F75, 324-331(2019). DOI: 10.1107/S2053230X19002796
2018
Degtjarik O., Demo G., Wimmerova M., Kuta Smatanova I.: Chapter 11: X-ray Crystallography. In Plant Structural Biology: Hormonal Regulations. Pages 203-221. Edited by Hejatko J. and Hakoshima T. ISBN 978-3-319-91351-3. Springer IP AG part of Springer Nature (2018)
2017
Sviridova E., Rezacova P., Bondar A., Veverka V., Novak P., Schenk G., Svergun D.I., Kuta Smatanova I. and Bumba L.: Structural basis of the interaction between the putative adhesion-involved and iron-regulated FrpD and FrpC proteins of Neisseria meningitides. Sci. Rep. 7, pages 14, 40408 (2017). DOI: 10.1038/srep40408.
2016
Brezovsky* J., Babkova* P., Degtjarik O., Fortova A., Gora A., Iermak I., Rezacova P., Dvorak P., Kuta Smatanova I., Prokop Z., Chaloupkova R. and Damborsky J.: Engineering a de novo transport tunnel. ACS Catalysis 6, 7597-7610 (2016). DOI: 10.1021/acscatal.6b02081
Degtjarik O., Brynda J., Ettrichova O., Kuty M., Sinha D., Kuta Smatanova I., Carey J., Ettrich R., Řeha D.: Quantum calculations indicate effective electron transfer between FMN and benzoquinone in a new crystal structure of E. coli WrbA. Journal of Physical Chemistry B120 (22): 4867–4877 (2016). DOI: 10.1021/acs.jpcb.5b11958
2015
Einspahr H., Kuta Smatanova I., Betzel Ch. and Mesters J.: Introduction to selected articles from the 15th ICCBM. Acta Crystallographica Section F, Volume 71, Issue 7, page 805 (2015). DOI: 10.1107/S2053230X15012534
Iermak, I., Degtjarik, O., Steffler, F., Sieber, V. and Kuta Smatanova, I.: Crystallization behaviour of glyceraldehyde dehydrogenase from Thermoplasma acidophilum. Acta Cryst. F71, Volume 71, Part 12, pages. 1475-1480 (2015). DOI:10.1107/S2053230X15020270
Csefalvay E, Lapkouski M, Guzanova A, Csefalvay L, Baikova T, Shevelev I, Bialevich V, Shamayeva K, Janscak P, Kuta Smatanova I, Panjikar S, Carey J, Weiserova M, Ettrich R: Functional Coupling of Duplex Translocation to DNA Cleavage in a Type I Restriction Enzyme. PLoS ONE 10(6): e0128700 (2015). DOI:10.1371/journal.pone.0128700
Liskova V., Bednar D., Prudnikova T., Rezacova P., Koudelakova T., Sebestova E., Kuta Smatanova I., Brezovsky J., Chaloupkova R., Damborsky J.: Balancing the stability-activity trade-off by fine-tuning dehalogenase access tunnels. ChemCatChem 7, 648 –659 (2015). DOI: 10.1002/cctc.201402792
2014
Chaloupkova R., Prudnikova T., Rezacova P., Prokop Z., Koudelakova T., Daniel L., Brezovsky J., Ikeda-Ohtsubo W., Sato Y., Kuty M., Nagata Y., Kuta Smatanova I., Damborsky J.: Structural and functional analysis of a novel haloalkane dehalogenase with two halide-binding sites, Acta Cryst. D70, 1884–1897 (2014). DOI:10.1107/S1399004714009018
Sykora J., Brezovsky J., Koudelakova T., Lahoda M., Fortova A., Chernovets T., Chaloupkova R., Stepankova V., Prokop Z., Kuta Smatanova I., Hof M., Damborsky J.: Dynamics and hydration explain failed functional transformation in dehalogenase design, Nature Chemical Biology, Vol. 10, No. 6, s. 428-430 (2014). ISSN 1552-4450, DOI:10.1038/nchembio.1502
Bumba L., Sviridova E., Kutá Smatanová I., Řezáčová P., Veverka V.: Backbone resonance assignments of the outer membrane lipoprotein FrpD from Neisseria meningitidis. Biomol NMR Assign 8, Issue 1, 53-55 (2014). ISSN 1874-2718, Published online 09 December 2012, DOI: 10.1007/s12104-012-9451-5
Lahoda M., Mesters J.R., Stsiapanava A., Chaloupkova R., Kuty M., Damborsky J., Kuta Smatanova I.: Crystallographic analysis of 1,2,3–trichloropropane biodegradation by haloalkane dehalogenase DhaA31. Acta Cryst. D70, 209-217 (2014). DOI:10.1107/S1399004713026254
2013
Kishko I., Carey J., Reha D., Brynda J., Winkler R., Harish B., Guerra R., Ettrichova O., Kukacka Z., Sheryemyetyeva O., Novak P., Kuty M., Kuta Smatanova I., Ettrich R, Lapkouski M.: 1.2 Å resolution crystal structure of Escherichia coli WrbA holoprotein. Acta Cryst. D69, Part 9, pages 1748-1757 (2013). DOI:10.1107/S0907444913017162
Tratsiak K., Degtjarik O., Drienovska I., Chrast L., Rezacova P., Kuty M., Chaloupkova R., Damborsky J., Kuta Smatanova I.: Crystallographic analysis of new psychrophilic haloalkane dehalogenases: DpcA from Psychrobacter cryohalolentis K5 and DmxA from Marinobacter sp. ELB17. Acta Cryst. F69, 683-688 (2013). DOI:10.1107/S1744309113012979
Degtjarik O., Chaloupkova R., Rezacova P., Kuty M., Damborsky J., Kuta Smatanova I.: Differences in crystallization of two LinB variants from Sphingobium japonicum UT26. Acta Cryst F69, Part 3, 284-287 (2013). DOI:10.1107/S1744309113002467
Degtjarik O., Dopitova R., Puehringer S., Nejedla E., Kuty M., Weiss M., Hejatko J., Janda L., Kuta Smatanova I.: Cloning, Expression, Purification, Crystallization and Preliminary X-ray Diffraction Analysis of AHP2, a signal transmitter protein from Arabidopsis thaliana. Acta Cryst F69, 158-161 (2013). DOI:10.1107/S174430911205186X
Koudelakova, T., Chaloupkova, R., Brezovsky, J., Prokop, Z., Sebestova, E., Hesseler, M., Khabiri, M., Plevaka, M., Kulik, D., Kuta Smatanova, I., Rezacova, P., Ettrich, R., Bornscheuer, U. T., Damborsky, J.: Engineering Enzyme Stability and Resistance to Organic Co-solvent by Access Tunnel Modification. Angewandte Chemie International Edition 52, 1959-1963 (2013). DOI: 10.1002/anie.201206708
2012
Kopecky V Jr, Kohoutova J, Lapkouski M, Hofbauerova K, Sovova Z, Ettrichova, O., Gonzales-Perez, S., Dulebo, A., Kaftan, D., Kuta Smatanova, I., Revuelta, J.L., Arellano, J.B., Carey, J., Ettrich R.: Raman Spectroscopy Adds Complementary Detail to the High-Resolution X-Ray Crystal Structure of Photosynthetic PsbP from Spinacia oleracea. PLoS ONE 7(10): e46694 (2012). DOI:10.1371/journal.pone.0046694
Nemcovicova I. and Kuta Smatanova I.: Chapter 11: Alternative Crystallization Technique: Cross Influence Procedure (CIP). In the Crystallization and Materials Science of Modern Artificial and Natural Crystals, Pages 249-276, Edited by: Elena Borisenko, ISBN 978-953-307-608-9, Publisher: InTech (January 2012)
2011
Lahoda M., Chaloupkova R., Stsiapanava A., Damborsky J., Kuta Smatanova I.: Crystallization and crystallographic analysis of the Rhodococcus rhodochrous NCIMB 13064 mutant DhaA31 and its complex with 1, 2, 3-trichloropropane. Acta Cryst F67, 397-400 (2011). DOI: 10.1107/S1744309111001254
Stsiapanava A., Chaloupkova R., Fortova A., Brynda J., Weiss M., Damborsky J., Kuta Smatanova I.: Crystallization and preliminary X-ray diffraction analysis of the wild type haloalkane dehalogenase DhaA and the variant DhaA13 complexed with different ligands. Acta Cryst F67, 253-257 (2011). DOI: 10.1107/S1744309110051286
Prudnikova, T., Chaloupkova, R., Sato, Y., Nagata, Y., Degtjarik, O., Kuty, M., Rezacova, P., Damborsky, J., Kuta Smatanova, I.: Development of a Crystallization Protocol for the DbeA1 Variant of Novel Haloalkane Dehalogenase from Bradyrhizobium elkani USDA94. Crystal Growth and Design 11, 516-519 (2011). DOI: 10.1021/cg1013363
2010
Sviridova E., Bumba L., Řezacova P., Prochazkova K., Kavan D., Bezouska K., Kuty M., Sebo P., Kuta Smatanova I.: Crystallization and preliminary crystallographic characterization of the iron-regulated outer membrane lipoprotein FrpD from Neisseria meningitidis. Acta Cryst F66, 1119-1126 (2010). DOI: 10.1107/S174430911003215X
Stsiapanava, A., Dohnalek, J., Gavira, J. A., Kuty, M., Koudelakova, T., Damborsky, J. and Kuta Smatanova, I.: Atomic resolution studies of haloalkane dehalogenases DhaA04, DhaA14 and DhaA15 with engineered access tunnels. Acta Cryst D66, 962-969 (2010). DOI: 10.1107/S09074449100027101
Prudnikova T., Gavira J.A., Řezáčová P., Pineda Molina E., Hunalová I., Sviridova E., Shmidt V., Kohoutová J., Kutý M., Kaftan D., Vácha F., García-Ruiz J.M., Kutá Smatanová I.: Towards the crystallization of photosystem II core complex from Pisum sativum L. Cryst. Growth Des. 10 (8) 3391-3396 (2010). DOI: 10.1021/cg901593x
2009
Klvana M.; Pavlova M.; Koudelakova T.; Chaloupkova R.; Dvorak P.; Stsiapanava A.; Kuty M.; Kuta-Smatanova I.; Dohnalek J.; Kulhanek P.; Wade R.C.; Damborsky J.: Pathways and Mechanisms for Product Release in the Engineered Haloalkane Dehalogenases Explored using Classical and Random Acceleration Molecular Dynamics Simulations. J. Mol. Biol. 392, 1339-1356 (2009). DOI:10.1016/j.jmb.2009.06.076
Wolfova J., Kuta Smatanova I., Brynda J., Mesters J.R., Lapkouski M., Kuty M., Natalello A., Chatterjee N., Chern S-Y., Ebbel E., Ricci A., Grandori R., Ettrich R., Carey J.: Structural organization of WrbA in apo- and holoprotein crystals. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1794, 1288-1298 (2009). DOI: 10.1016/j.bbapap.2009.08.001. (The cover picture of this issue was selected from our paper)
Kohoutová J., Kopecký Jr. V., Lapkouski M., Hofbauerová K., Sovová Ž, González-Pérezc S., Kutá Smatanová I., Revuelta J.L., Arellano J.B., Ettrich R.: Structural analysis of extrinsic PsbP protein of PSII from Spinacea oleracea and its interaction with the oxygen-evolving complex. FEBS Journal 276, 146-147 (2009).
Lapkouski M., Panjikar S., Janscak P., Kuta Smatanova I., Carey J., Ettrich R., Csefalvay E.: Structure of the motor subunit and translocation model for EcoR124I restriction-modification complex. Nature Structural & Molecular Biology 16(1), 94-5 (2009). Epub 2008 Dec 14. DOI: 10.1038/nsmb.1523
Prudnikova T., Mozga T., Rezacova R., Chaloupkova R., Sato Y., Nagata Y., Brynda J., Kuty M., Damborsky J. and Kuta Smatanova I.: Crystallisation and preliminary X-ray analysis of a novel haloalkane dehalogenase DbeA from Bradyrhizobium elkani USDA94. Acta Cryst. F65, 353-356 (2009). DOI: 10.1107/S1744309109007039
Kohoutová J., Kutá Smatanová I., Brynda J., Lapkouski M., Revuelta J.L., Arellano J.B., Ettrich R.: Crystallization and preliminary crystallographic characterization of the extrinsic PsbP protein of photosystem II from Spinacia oleracea. Acta Cryst. F65, 111-115 (2009). DOI: 10.1107/S1744309108040578
Wolfová J., Brynda J., Mesters J.R., Ettrich R., Carey J., Kutá Smatanová I.: Structural changes of tetrameric flavoprotein WrbA upon flavin binding. Materials Structure 16, 2a, k56-57 (2009).
2008
Wolfová J., Brynda J., Mesters J.R., Carey J., Grandori R. and Kutá Smatanová I.: Crystallographic study of Escherichia coli favoprotein WrbA, a new NAD(P)H-dependent guinine oxidoreductase. Materials Structure 15, 1, 55-57 (2008).
Stsiapanava A., Koudelakova T., Lapkouski M., Pavlova M., Damborsky J. and Kuta Smatanova I.: Crystals of DhaA mutants from Rhodococcus rhodochrous NCIMB 13064 diffracted to ultra high resolution: crystallization and preliminary diffraction analysis. Acta Cryst. F64, 137-140 (2008). DOI: 10.1107/S1744309108002066
2007-1997
Tomčová I. and Kutá Smatanová I.: Copper co-crystallization and divalent metal salts cross-influence effect – a new optimisation tool improving crystal morphology and diffraction quality. Journal of Crystal Growth 306, 383-389 (2007). DOI: 10.1016/j.jcrysgro.2007.05.054
Wolfová J., Mesters J.R., Brynda J., Grandori R., Natalello A., Carey J. and Kutá Smatanová I.: Crystallization and preliminary diffraction analysis of E. coli WrbA in complex with its cofactor flavin mononucleotide. Acta Cryst. F63, 571-575 (2007). DOI: 10.1107/S1744309107026103
Lapkouski M., Panjikar S., Kuta Smatanova I. and Csefalvay E.: Purification, crystallization and preliminary X-ray analysis of the HsdR subunit of the EcoR124I endonuclease from E. coli. Acta Cryst. F63, 582-585 (2007). DOI: 10.1107/S174430910702622X
Carey J., Brynda J., Wolfová J., Grandori R., Gustavsson T., Ettrich R.H., Kutá Smatanová I.: WrbA bridges bacterial flavodoxins and eukaryotic NAD(P)H:quinone oxidoreductases. Protein Science 16, 10, 2301-2305 (2007). DOI: 10.1110/ps.073018907. (The cover picture of this issue was selected from our paper)
Tomčová I. and Kutá Smatanová I.: Cross-crystallization as a new optimization tool of crystallization procedures. Materials Structure 14, 1, 3-5 (2007). (The cover picture of this issue was selected from our paper)
Prudnikova T., Kutý M., Gavira J.A., Palenčár P., Vácha F., Řezáčová P., Garcia-Ruiz J.M. and Kutá Smatanová I.: Crystallization and structure-functional study of the photosystem II from higher plants. Materials Structure 14, 1, 5-7 (2007).
Kutá Smatanová I., Gavira J.A., Řezáčová P., Vácha F., García-Ruiz J.M.: New techniques for membrane protein crystallization tested on photosystem II core complex of Pisum sativum. Photosynthesis Research 90 (3), 255-259 (2006). DOI: 10.1007/s11120-007-9131-y
Tomčová I., Mamede Branca R.M., Bodó G., Bagyinka C. and Kutá Smatanová I.: Cross-crystallization and preliminary diffraction analysis of a novel di-heme cytochrome c4. Acta Cryst. F62, 820-824 (2006). DOI: 10.1107/S1744309106027710
Wolfova J., Grandori R., Kozma E., Chatterjee N., Carey J. and Kuta Smatanova I.: Crystallization of the flavoprotein WrbA optimized by using additives and gels. Journal of Crystal Growth 284, 3-4, 502-505 (2005). DOI: 10.1016/j.jcrysgro.2005.07.043
Hogg T., Kuta Smatanova I., Bezouska K., Ulbrich N. and Hilgenfeld R.: Sugar-mediated lattice contacts in crystals of a plant glycoprotein. Acta Cryst. D58, 1734-1739 (2002). DOI: 10.1107/S0907444902014506
Oakley A.J., Prokop Z., Bohac M., Kmunicek J., Jedlicka T., Monincova M., Kuta Smatanova I., Nagata Y., Damborsky J., Wilce M.C.J.: Exploring the structure and activity of Haloalkane Dehalogenase from Sphingomonas paucimobilis UT26: Evidence for product and water mediated inhibition. Biochemistry 41, 4847-4855 (2002). DOI: 10.1021/bi015734i
Marek, J., Vevodova, J., Kuta Smatanova, I., Nagata, J., Svensson, L.A, Newman, J., Takagi, M. and Damborsky, J.: Crystal Structure of the Haloalkane Dehalogenase from Sphingomonas paucimobilis UT26. Biochemistry 39, 14082-14086 (2000). DOI: 10.1021/bi001539c
Schwendt, P., Svancarek, P., Smatanova, I. and Marek, J.: Stereospecific formation of alpha-Hydroxylato Oxo Peroxo Complexes of Vanadium(V). Crystal Structure of (NBu4)2[V2 O2(O2)2 (L-lact)2].2H2O and (NBu4)2[V2 O2(O2)2 (D-lact)(L-lact)].2H2O. Journal of Inorganic Biochemistry 80, 59-64 (2000). DOI: 10.1016/S0162-0134(00)00040-4
Smatanova, I.K., Marek, J., Svancarek, P., Schwendt, P. :Bis(tetra-n-butylammonium)Bis[(mandelato)oxo-(peroxo)vanadate(V)] mandelic acid solvate. Acta Cryst. C56, 154-155 (2000). DOI: 10.1107/S0108270199013475
Svancarek, P., Schwendt, P., Tatiersky, J., Smatanova, I. and Marek, J.: Oxo Peroxo Glycolato Complexes of Vanadium (V). Crystal Structure of (NBu4)2[V2O2(O2)2(C2H2O3)2].H2O. Monatshefte für Chemie 131, 145-154 (2000). DOI: 10.1007/PL00010301
Smatanova, I., Nagata, Y., Svensson, L. A., Takagi, M. and Marek, J.: Crystallization and preliminary X-ray diffraction analysis of haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26. Acta Cryst. D55, 1231-1233 (1999). DOI: 10.1107/S090744499900459X
Smatanova, I., Marek, J., Svancarek, P., Schwendt, P. : Bis(tetra-n-butylammonium) Bis[(methyllactato) dioxo-vanadate(V)] Dihydrate. Acta Cryst. C54, 1249 1251 (1998). DOI: 10.1107/S0108270198003424
Svancarek, P., Smatanova, I., Schwendt, P., Marek, J.: Vanadium (V) peroxo complexes with alpha-hydroxycarboxylates as heteroligands. Chemické listy, 91 (9), 632-632 (1997).